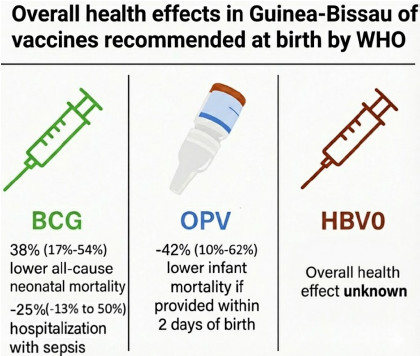
We are pleased to share the protocol for the upcoming trial, which will compare the currently recommended neonatal vaccine schedule of BCG + OPV vaccines to the future policy of BCG + OPV + HBV0, in regard to non-specific effects.
We have compiled answers to frequently asked questions in a list. You can find it here.
The feedback that we have received is that this is an important trial which can impact future vaccine policies in the region and worldwide. However, a lot of misinformation has also circulated. We address this here.
The feedback that we have received is that this is an important trial which can impact future vaccine policies in the region and worldwide.
The Danish-Guinean Bandim Health Project functions under the Ministry of Health of Guinea-Bissau and has conducted health research within vaccines, TB, HIV and malaria since 1978, shortly after Guinea-Bissau’s independence. Our core mission is to reduce child mortality, with emphasis on the beneficial non-specific effects of live vaccines, which was discovered in Guinea-Bissau and has since been confirmed epidemiologically and immunologically by other research groups.
Developing countries are generally deprived of research and even more so of randomized trials, which are a key method of producing the data that will help optimize the use and timing of infant vaccines. This trial bears relevance to the newborns that will be enrolled and similar countries in the region, which we are deeply embedded in and dedicated to.
We are aware of ongoing polemic in the US and will stress here that this trial has no connection with domestic US vaccination policies. There is a plethora of misinformation and rumours circulating, including that the trial has started and that it has been cancelled. None of this is true.
Preparations of the trial are ongoing and we will announce when it has started.
